ImmunOs’ HLA-based pipeline includes first-in-class immunotherapies for the treatment of cancer and autoimmune disease. The Company’s immuno-oncology pipeline is based on its ability to engage both the innate and the adaptive immune systems with single, multitasking molecules that remodel the tumor microenvironment, enhancing anti-tumor immunity and resulting in the killing of tumor cells.
The Company´s lead product candidate, IOS-1002, is an HLA-based protein which blocks the activation of three different immunosuppressive receptors (also known as checkpoints) on innate and adaptive immune cells: LILRB1 (ILT2), LILRB2 (ILT4), and KIR3DL1.
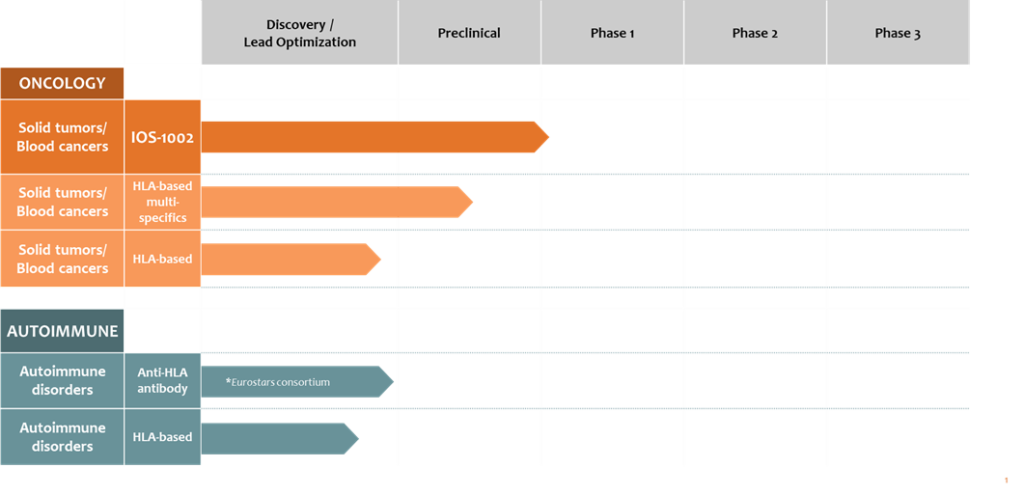
LEAD IMMUNO-ONCOLOGY PROGRAM IOS-1002
The Company´s lead program, IOS-1002 (formerly iosH2), binds to and blocks the activation of specific leukocyte immunoglobulin-like receptors (LILRBs, also known as ILTs, i.e., immunoglobulin-like transcripts) and killer cell immunoglobulin-like receptors (KIRs) and has the potential to treat multiple types of solid tumors and hematologic malignancies. Specifically, IOS-1002 binds to LILRB1 (ILT2), LILRB2 (ILT4), and KIR3DL1 receptors on innate and adaptive immune cells that suppress immune responses when activated. By blocking this activation, IOS-1002 alters the tumor microenvironment to induce tumor infiltration of M1 macrophages, T cells and NK cells resulting in the killing of tumor cells. IOS-1002 is designed as a potential monotherapy and as a combination therapy to improve medical outcomes in non-responders and patients that have progressed on adaptive immunity agents. IOS-1002 is differentiated from competitor approaches as it targets multiple LILRB receptors and a KIR receptor instead of a single checkpoint receptor.
In various animal models, IOS-1002 has led to tumor shrinkage (up to 90%) and to improved survival both as a monotherapy and in combination therapy.
ImmunOs has successfully completed non-clinical efficacy, pharmacokinetic and safety studies to support a regulatory filing for IOS-1002 in late 2022. A first in human clinical trial of IOS-1002 has been designed to deliver proof-of-concept data, initially in a dose escalation trial, and subsequently in expansion cohorts as a monotherapy and in combination with anti-PD1 treatment (e.g., pembrolizumab).
LEAD AUTOIMMUNE DISEASE PROGRAM
The lead program in ImmunOs´ autoimmune pipeline is an antibody targeting specific HLA molecules associated with autoimmune diseases.